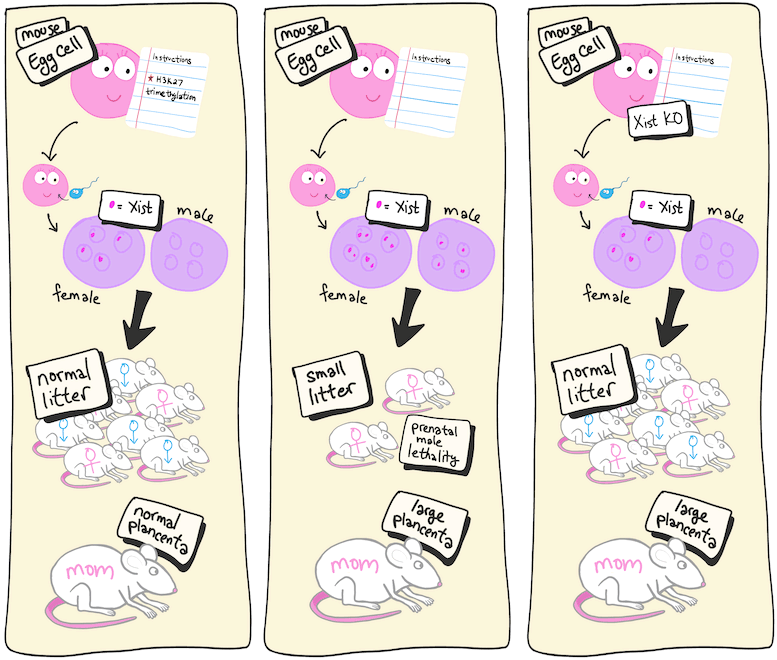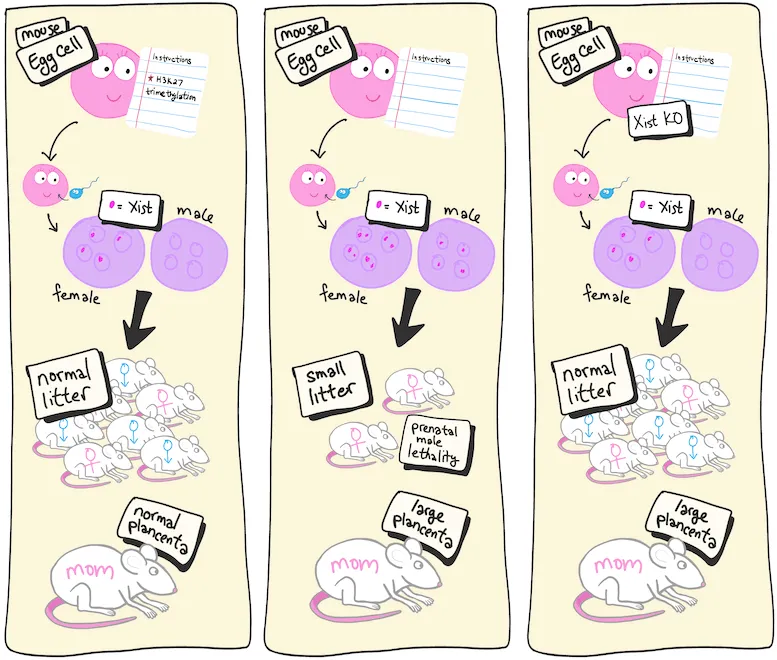
Représentation cartographique de la première expérience. (A gauche) La situation normale chez les souris de type sauvage. (Au milieu) Lorsque les ovules ne contiennent pas de triméthylation H3K27, le Xist maternel est actif et létal pour les mâles. (Droite) Un knockout (KO) supplémentaire de Xist maternel prévient la mort prénatale (fausses couches) chez les mâles. Le KO supplémentaire d’autres gènes qui n’ont pas réussi à s’imprimer sauve le placenta maternel élargi (non représenté). Crédit : RIKEN
Un gène responsable de la mort prénatale lorsque des instructions transgénérationnelles critiques manquent dans les ovules a été découvert par des chercheurs du Centre RIKEN pour les sciences médicales intégratives (IMS) au Japon, dirigés par Azusa Inoue.
“Cette étude a identifié des gènes essentiels au développement du fœtus dont l’expression est contrôlée par des modifications d’histones transmises des œufs à la génération suivante”, explique Inoue. “Ces résultats ont des implications pour la compréhension de l’infertilité et le développement de traitements”.
Pour que les embryons se développent normalement, les ovules et les spermatozoïdes doivent d’abord acquérir des instructions biologiques essentielles avant de se rencontrer. Lorsqu’un ovule est fécondé, certaines de ces instructions indiquent aux gènes s’ils doivent être activés ou désactivés, selon qu’ils proviennent de la mère ou du père. Ce processus est connu sous le nom d’empreinte génomique et fait l’objet de la nouvelle étude.
Lorsque les modifications de l’expression des gènes sont transmises à la génération suivante, on parle de changements épigénétiques transgénérationnels car ce sont des changements héritables même si le DNA code remains unchanged. Inoue and his team have been studying a specific set of transgenerational epigenetic instructions given to egg cells called histone H3 lysine 27 (H3K27) trimethylation. In previous studies, they found that preventing these instructions led to prenatal death, particularly for male embryos, and also to enlarged placentas in the mothers. The new study asked whether those outcomes were directly related to failed imprinting.
The study began by knocking out a gene required for H3K27 trimethylation in eggs so that the transgenerational instructions could not be given. Next, the team added a knockout of the Xist gene to these eggs. Because the male offspring tended to die, the researchers suspected that the culprit was a gene on the sex chromosome. As it turns out, there are nine maternal genes known to be suppressed in embryos in favor of the ones with paternal origins. And only one, Xist, is on the X-chromosome.
The results were almost as expected. Prenatal death was greatly reduced, and the male-skewed lethality was gone after knocking out Xist. This showed that failed Xist imprinting was the reason for the prenatal death. However, the placenta was still enlarged. Reasoning that this was likely related excess expression of the other eight genes that failed to imprint, the team created eight different deletion mutants in the double knockout embryos. They found that for three of the genes, this resulted in normal-sized placentas.
“We succeeded in curing developmental defects in a mouse model that otherwise suffers from prenatal lethality and placental malformation due to the lack of transgenerational epigenetic instructions from mothers,” says Inoue. The researchers plan to conduct more experiments to determine how these specific biological instructions are established when egg cells are created, and whether environmental factors can influence the process.
Reference: “Noncanonical imprinting sustains embryonic development and restrains placental overgrowth” by Shogo Matoba, Chisayo Kozuka, Kento Miura, Kimiko Inoue, Mami Kumon, Ryoya Hayashi, Tatsuya Ohhata, Atsuo Ogura and Azusa Inoue, 28 April 2022, Genes & Development.
DOI: 10.1101/gad.349390.122