Josh Portner, étudiant diplômé de l’Université de Chicago, recueille des données sur la diffusion des rayons X à partir de minuscules “supercristaux”. Les scientifiques espèrent que ces supercristaux pourront constituer la base de nouvelles technologies grâce à une nouvelle méthode permettant de les faire communiquer entre eux électroniquement. Crédit : Laboratoire Talapin/Université de Chicago
Les scientifiques rasent les “cheveux” des nanocristaux pour améliorer leurs propriétés électroniques.
La percée des chimistes de l’Université de Chicago pourrait donner naissance à des dispositifs futurs tels que les écrans et les cellules solaires de nouvelle génération.
Vous pouvez aujourd’hui transporter un ordinateur entier dans votre poche car les blocs de construction technologiques sont de plus en plus petits depuis les années 1950. Mais pour créer les futures générations d’appareils électroniques – tels que des téléphones plus puissants, des cellules solaires plus efficaces ou même des ordinateurs quantiques – les scientifiques devront mettre au point des technologies entièrement nouvelles à des échelles très réduites.
L’un des domaines d’intérêt est celui des nanocristaux. Ces minuscules cristaux peuvent s’assembler en de nombreuses configurations, mais les scientifiques ont eu du mal à trouver le moyen de les faire communiquer entre eux.
Une nouvelle étude présente une percée pour faire fonctionner ensemble électroniquement des nanocristaux. Publié le 24 mars 2022 dans le journal Science, la recherche pourrait ouvrir les portes à de futurs dispositifs dotés de nouvelles capacités.
“Nous appelons ces blocs de construction super atomiques, car ils peuvent accorder de nouvelles capacités – par exemple, permettre aux caméras de voir dans la gamme infrarouge”, a déclaré University of Chicago Prof. Dmitri Talapin, the corresponding author of the paper. “But until now, it has been very difficult to both assemble them into structures and have them talk to each other. Now for the first time, we don’t have to choose. This is a transformative improvement.”
In their paper, the scientists lay out design rules which should allow for the creation of many different types of materials, said Josh Portner, a Ph.D. student in chemistry and one of the first authors of the study.
A tiny problem
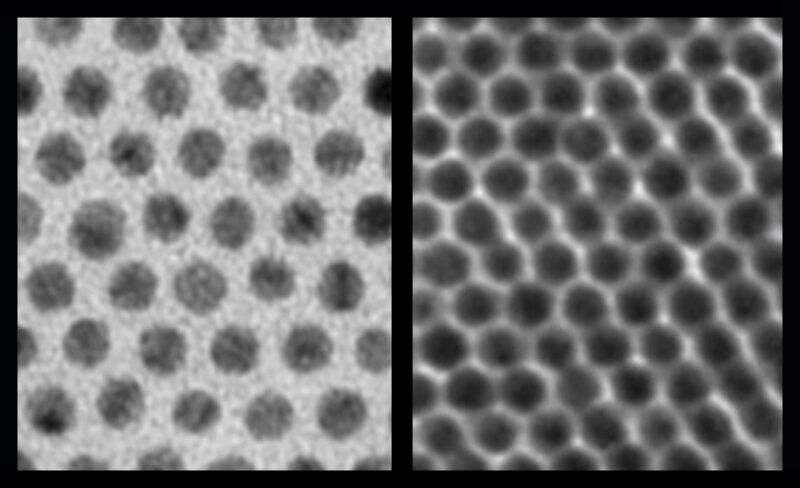
Scientists can grow nanocrystals out of many different materials: metals, semiconductors, and magnets will each yield different properties. But the trouble was that whenever they tried to assemble these nanocrystals together into arrays, the new supercrystals would grow with long “hairs” around them.
These hairs made it difficult for electrons to jump from one nanocrystal to another. Electrons are the messengers of electronic communication; their ability to move easily along is a key part of any electronic device.
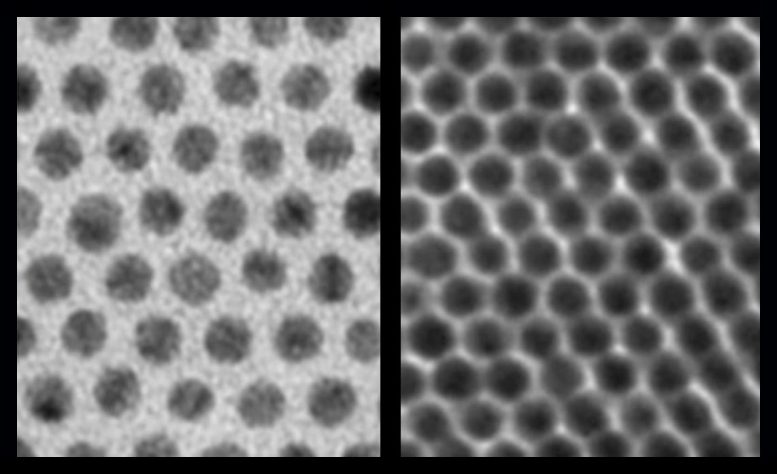
Previously, when scientists assembled nanocrystals together into arrays, they would grow with long “hairs” around them (see left image, taken by an electron microscope).These hairs made it difficult for electrons to jump from one nanocrystal to another. But a new technique reduces the hairs around each nanocrystal (right image) to pack them in more tightly and improve the electronic communication. Credit: Talapin lab/University of Chicago
The researchers needed a method to reduce the hairs around each nanocrystal, so they could pack them in more tightly and reduce the gaps in between. “When these gaps are smaller by just a factor of three, the probability for electrons to jump across is about a billion times higher,” said Talapin, the Ernest DeWitt Burton Distinguished Service Professor of Chemistry and Molecular Engineering at UChicago and a senior scientist at Argonne National Laboratory. “It changes very strongly with distance.”
To shave off the hairs, they sought to understand what was going on at the atomic level. For this, they needed the aid of powerful X-rays at the Center for Nanoscale Materials at Argonne and the Stanford Synchrotron Radiation Lightsource at SLAC National Accelerator Laboratory, as well as powerful simulations and models of the chemistry and physics at play. All these allowed them to understand what was happening at the surface—and find the key to harnessing their production.
Part of the process to grow supercrystals is done in solution—that is, in liquid. It turns out that as the crystals grow, they undergo an unusual transformation in which gas, liquid and solid phases all coexist. By precisely controlling the chemistry of that stage, they could create crystals with harder, slimmer exteriors which could be packed in together much more closely. “Understanding their phase behavior was a massive leap forward for us,” said Portner.
The full range of applications remains unclear, but the scientists can think of multiple areas where the technique could lead. “For example, perhaps each crystal could be a qubit in a quantum computer; coupling qubits into arrays is one of the fundamental challenges of quantum technology right now,” said Talapin.
“This is a transformative improvement.”
— Prof. Dmitri Talapin
Portner is also interested in exploring the unusual intermediate state of matter seen during supercrystal growth: “Triple phase coexistence like this is rare enough that it’s intriguing to think about how to take advantage of this chemistry and build new materials.”
The study included scientists with the University of Chicago, Technische Universität Dresden, Northwestern University, Arizona State University, SLAC, Lawrence Berkeley National Laboratory, and the University of California, Berkeley.
The research was conducted in part at the DOE’s Advanced Materials for Energy-Water Systems Center, the Midwest Integrated Center for Computational Materials, the Center for Nanoscale Materials at Argonne, and the Stanford Synchrotron Radiation Lightsource at SLAC National Accelerator Laboratory.
Reference: “Self-assembly of nanocrystals into strongly electronically coupled all-inorganic supercrystals” by Igor Coropceanu, Eric M. Janke, Joshua Portner, Danny Haubold, Trung Dac Nguyen, Avishek Das, Christian P. N. Tanner, James K. Utterback, Samuel W. Teitelbaum, ¸ Margaret H. Hudson, Nivedina A. Sarma, Alex M. Hinkle, Christopher J. Tassone, Alexander Eychmüller, David T. Limmer, Monica Olvera de la Cruz, Naomi S. Ginsberg and Dmitri V. Talapin, 24 March 2022, Science.
DOI: 10.1126/science.abm6753
Funding: U.S. Department of Energy, U.S. Department of Defense, National Science Foundation, Arnold and Mabel Beckman Foundation, Alfred P. Sloan Foundation, David and Lucile Packard Foundation, Camille and Henry Dreyfus Teacher-Scholar Awards, Sherman Fairchild Foundation.