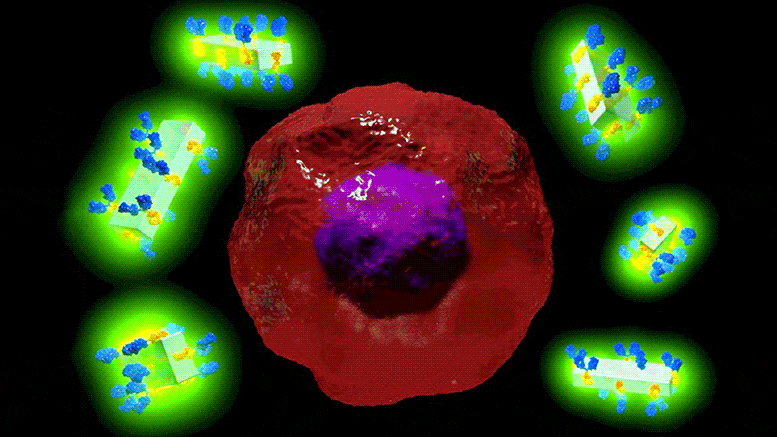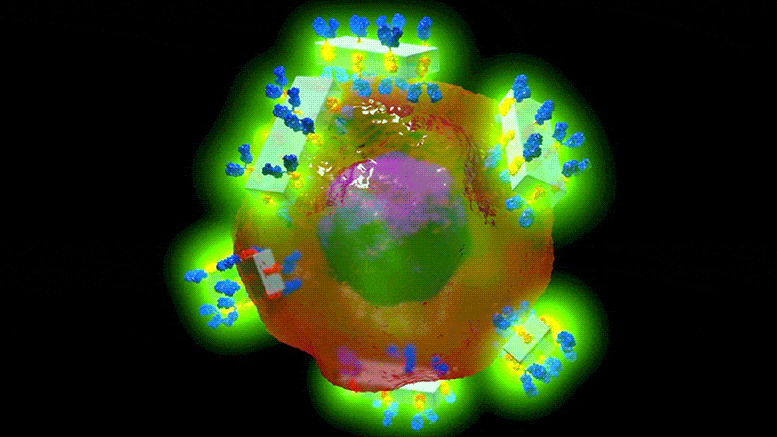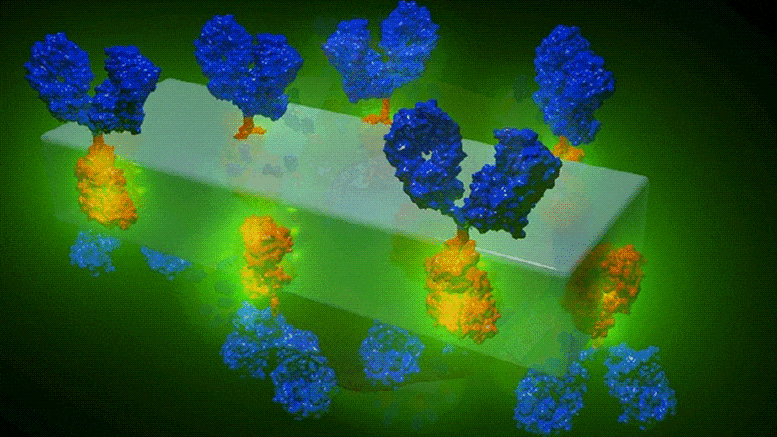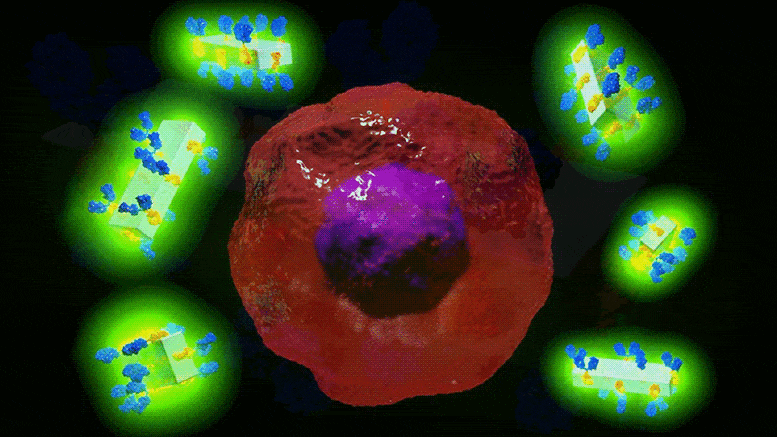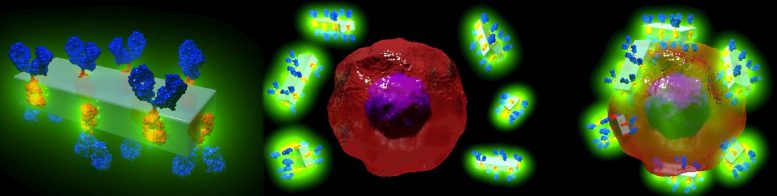
Illustration schématique des nouveaux cristaux d’anticorps MOF et de leur capacité à rechercher spécifiquement les cellules cancéreuses pour les détecter et délivrer des médicaments très puissants avec une précision sans précédent. Crédit : Dr Francesco Carraro et Prof. Paolo Falcaro (co-auteur principal et co-auteur principal de l’article sur les matériaux avancés).
Cela ressemble à de la science-fiction : un cristal artificiel qui peut être attaché à des anticorps et les surcharger avec des médicaments puissants ou des agents d’imagerie qui peuvent rechercher des cellules malades avec une précision extrême, ce qui entraîne moins d’effets indésirables pour le patient.
Or, c’est précisément ce que des chercheurs du Centre australien des maladies du sang de l’université Monash, en collaboration avec la TU Graz (Autriche), ont mis au point : le premier cadre métallo-organique (MOFs) antibody-drug delivery system that has the potential to fast-track potent new therapies for cancer, cardiovascular and autoimmune diseases.
The in vitro study showed that when MOF antibody crystals bind to their target cancer cells and if exposed to the low pH in the cells, they break down, delivering the drugs directly and solely to the desired area.
The metal-organic framework, a mixture of metal (zinc) and carbonate ions, and a small organic molecule (an imidazole, a colorless solid compound that is soluble in water) not only keeps the payload attached to the antibody but can also acts as a reservoir of personalized therapeutics. This is a benefit with the potential to become a new medical tool to target specific diseases with customized drugs and optimized doses.
The findings are now published in the world-leading journal Advanced Materials.
Co-senior author Professor Christoph Hagemeyer, Head of the NanoBiotechnology Laboratory at the Australian Centre for Blood Diseases, Monash University, says while more funding is needed to take the research into the next phase and to patients, the new method is cheaper, faster, and more versatile than anything available currently.
“The method offers the opportunity to personalize treatment and given the precision possible, may eventually change the current dosage needed for patients, resulting in fewer side effects and making treatments cheaper,” said Professor Hagemeyer.
Co-first author Dr. Karen Alt, Head of the Nano Theranostics Laboratory at the Australian Centre for Blood Diseases, Monash University, says: “With just 0.01 percent of chemotherapy currently reaching the cancer tissue, this revolutionary new method can boost the potency of the drugs reaching their target.”
“With over 80 different monoclonal antibodies approved for clinical use, this approach has enormous potential to improve these antibodies for the targeted delivery of diagnostic agents and therapeutic drugs. The goal is that ultimately the clinical translation of this technology will improve the quality of life for patients suffering from serious diseases,” said Dr. Alt.
Reference: “Self-Assembly of Oriented Antibody-Decorated Metal–Organic Framework Nanocrystals for Active-Targeting Applications” by Karen Alt, Francesco Carraro, Edwina Jap, Mercedes Linares-Moreau, Raffaele Riccò, Marcello Righetto, Marco Bogar, Heinz Amenitsch, Rania A. Hashad, Christian Doonan, Christoph E. Hagemeyer and Paolo Falcaro, 6 December 2021, Advanced Materials.
DOI: 10.1002/adma.202106607