La préférence pour la chiralité dans la matière vivante peut apparaître spontanément pour optimiser la récolte d’énergie.
Lorsqu’on tient une main droite devant un miroir, on peut voir l’image réfléchie de la main gauche, et vice versa. Louis Pasteur a observé en 1848 que les molécules organiques sont semblables aux mains humaines en ce sens qu’elles se présentent sous forme de paires de miroirs constitués de gauchers et de droitiers. Nous savons maintenant que cette chiralité (du mot grec signifiant “main”) est une caractéristique des molécules organiques.
Les molécules organiques sont riches en atomes de carbone, qui forment des liens pour créer une “nanomain” droite ou gauche. Pourtant, la vie choisit presque toujours d’utiliser exclusivement l’un des deux jumeaux, un phénomène connu sous le nom d’homochiralité. La vie terrestre, par exemple, est basée sur des gauchers
;” data-gt-translate-attributes=”[{” attribute=””>amino acids and right-handed sugars.
While many explanations were suggested, how and why homochirality emerged remains an enigma. Chiral symmetry breaking, which is a phenomenon where a 50-50 ratio mixture of left and right-handed molecules departs to favor one over the other, is of great research interest in biochemistry. Understanding the origin of homochirality is highly important for investigating the origin of life, as well as more practical applications such as the synthesis of chiral drug molecules.
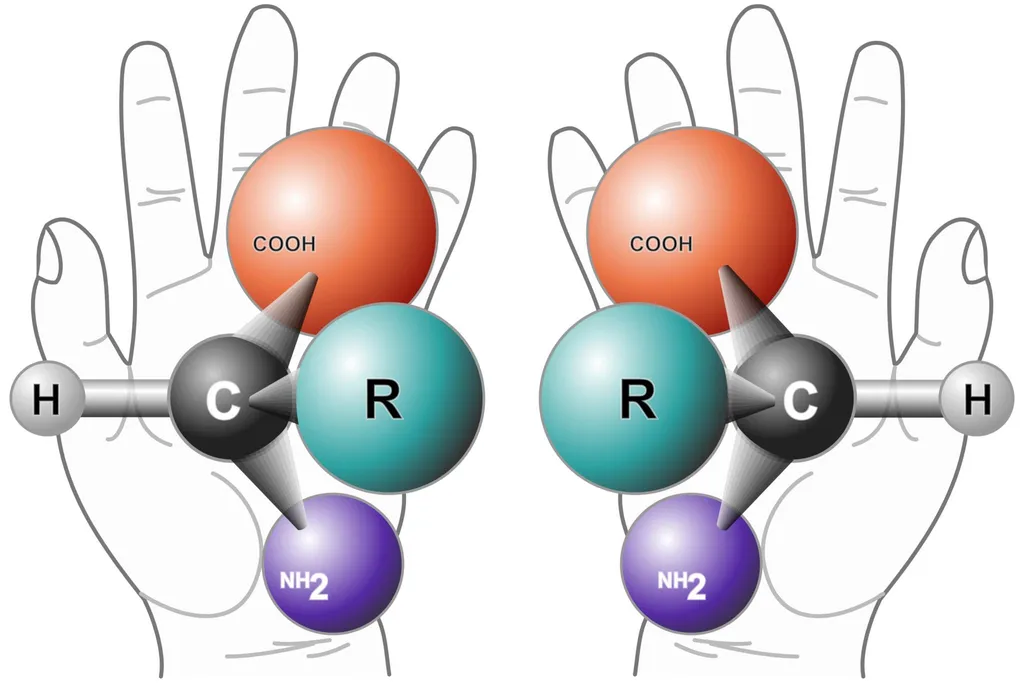
A “chiral” molecule is one that is not superposable with its mirror image. Like left and right hands that have a thumb, fingers in the same order, but are mirror images and not the same, chiral molecules have the same things attached in the same order, but are mirror images and not the same. Although most amino acids can exist in both left- and right-handed forms, Life on Earth is made of left-handed amino acids, almost exclusively.
A model proposes a novel explanation for the emergence of homochirality in life – a longstanding puzzle about the origin of life on Earth.
It is widely believed that life originated in habitats rich in energy sources – such as hydrothermal vents in the depths of primordial oceans. Considering possible primordial Earth scenarios, Prof. Tsvi Tlusty and Dr. William Piñeros from the Center for Soft and Living Matter within the Institute for Basic Science, South Korea, envisioned a complex network of chemical reactions that exchange energy with the environment. When the team used a mathematical model and system simulation to emulate a well-stirred solution of different chemical elements in a container, they surprisingly found out that such systems naturally tend to break the molecular mirror symmetry.
Homochirality emerges spontaneously in prebiotic chemical networks that adapt to optimize energy harvesting from the environment.
Previously it was believed that chiral symmetry breaking requires multiple loops of auto-catalysis, which increasingly produces one enantiomer of a molecule while inhibiting the formation of the other. However, the IBS team’s results showed that the underlying mechanism of symmetry breaking is very general, as it can occur in large reaction systems with many random molecules and does not require sophisticated network architectures. It was found that this sharp transition to homochirality stems from the self-configuration of the reaction network in order to achieve more efficient harvesting of energy from the environment.
The model developed by Piñeros and Tlusty showed that highly-dissipating systems and large energy differences are more prone to inducing chiral symmetry breaking. Furthermore, the calculations revealed that such transitions are almost inevitable, so it is reasonable to believe they may generically occur in random chemical reaction systems. Thus, the energy harvesting optimization-based model demonstrated by the group explains how homochirality could have spontaneously arisen from the harsh, energy-rich environment of the early planet Earth.
The proposed mechanism of symmetry breaking is a general one and can apply to other transitions in living matter that lead to increased complexity.
Moreover, the model proposes a general mechanism that explains how the complexity of a system can grow as it better adapts to exploit a varying environment. This suggests that chiral symmetry breaking is an inherent hallmark of any complex system (such as life) that is capable of configuring itself to adapt to an environment. These findings may furthermore explain spontaneous symmetry breakings in much more complex biological processes, such as cell differentiation and the emergence of new genes.
This study will be published today (April 26, 2022) in the journal Nature Communications.
Reference: “Spontaneous chiral symmetry breaking in a random driven chemical system” 26 April 2022, Nature Communications.
DOI: 10.1038/s41467-022-29952-8